With the assumption that the ammonium ion is formed from the reaction between ammonia and hydrogen io ,describe the formation of bond between these two species and state the type of bond formed?
1 Answer
The formation of the ammonium ion is an example of coordinate bonding.
Explanation:
To get a better understanding of how the ammonium ion,
The molecule has a total of 8 valence electrons, 5 from nitrogen and 1 from each of the three hydrogen atoms.
The nitogen atom will bond with the hydrogen atoms via single bonds. Notice that you have a lone pair of electrons present on the nitrogen atom.
Now, a hydrogen ion,
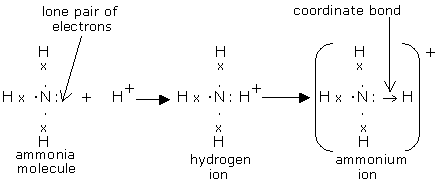
What you're essentially dealing with here is the reaction between a Lewis base, which an electron donor, and a Lewis acid, which acts as an electron acceptor.
Using other terminology, ammonia acts as a nucleophile, which is a reactant that provides a lone pair of electrons for the formation of a covalent bond.
The nitrogen atom donates its lone pair of electrons to form a coordinate bond with the proton. These two electrons are now bonding electrons between nitrogen and the fourth hydrogen atom in
The bond is called coordinate (or dative covalent) because both bonding electrons come from one of the atoms that form the bond. In this case, nitrogen contributes both bonding electrons, since the hydrogen ion has no electrons to begin with.

