Would cyclohexanone be a methyl ketone?
1 Answer
No. A methyl ketone has one methyl group and one
Do keep in mind though that cyclohexylacetone and cyclohexanone are not the same compounds. They might be easy to mix up visually, but by name, they are fairly different.
A methyl ketone and a cyclohexanone structure look like this:
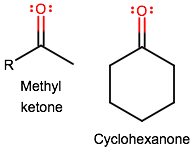
One could argue that cyclohexanone doesn't count as a methyl ketone since a methyl ketone requires a methyl group on one side and an
We could go on and say that a methyl ketone has a carbonyl group that is necessarily not directly connected onto any ring structure, if the
For instance, cyclohexylacetone (or cyclohexylethanone, or cyclohexyl methyl ketone) is a methyl ketone:
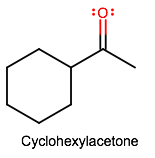
And you can see that the carbonyl is not directly connected onto the ring; it's separated by one carbon-carbon bond.

