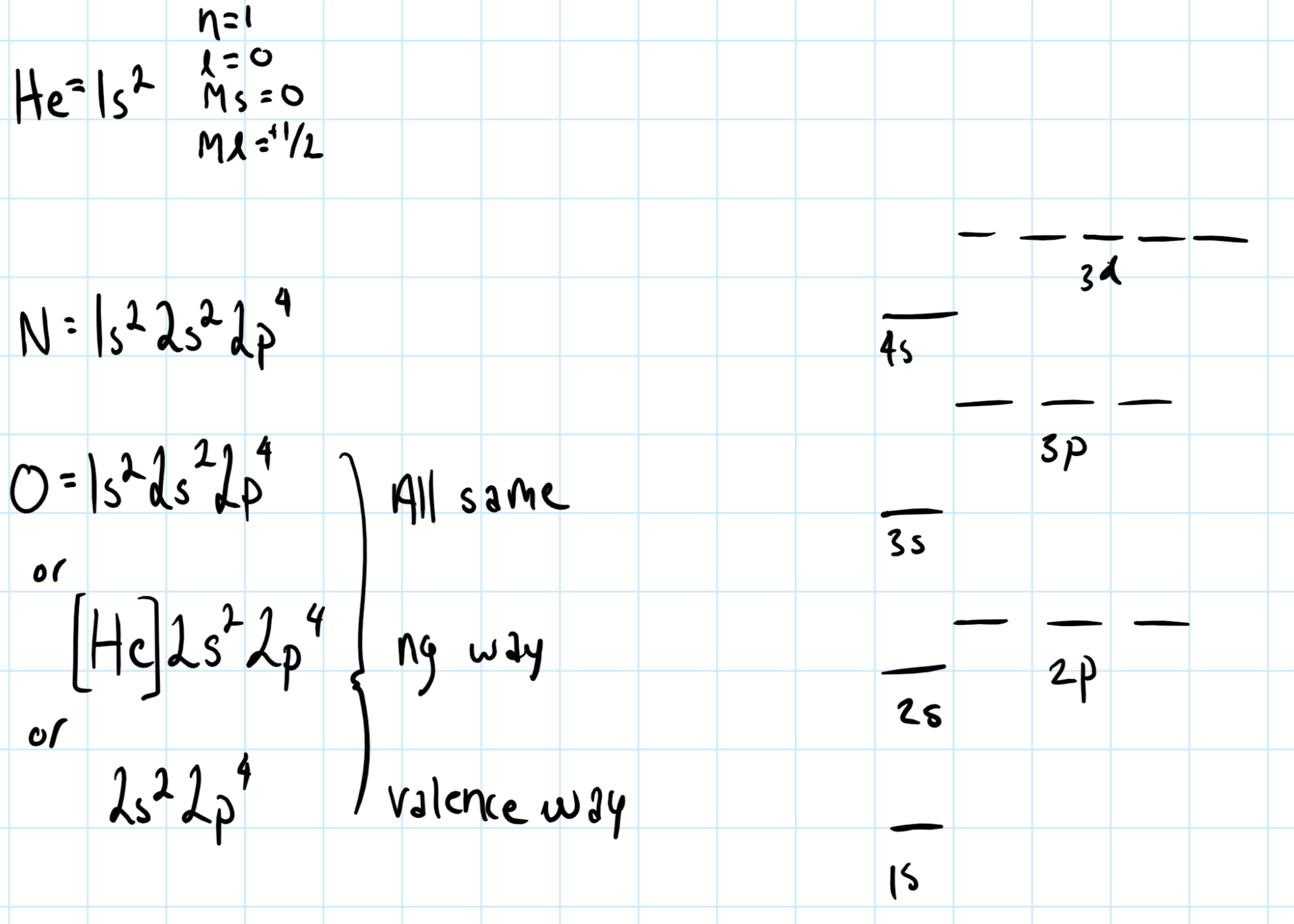Would these orbital descriptions be atomic or hybrid orbitals? s, p, d, f, #sp^2#, #sp^3#
1 Answer
s,p,d,f are usually used to describe atomic orbitals.
Explanation:
Consider the hybridization of
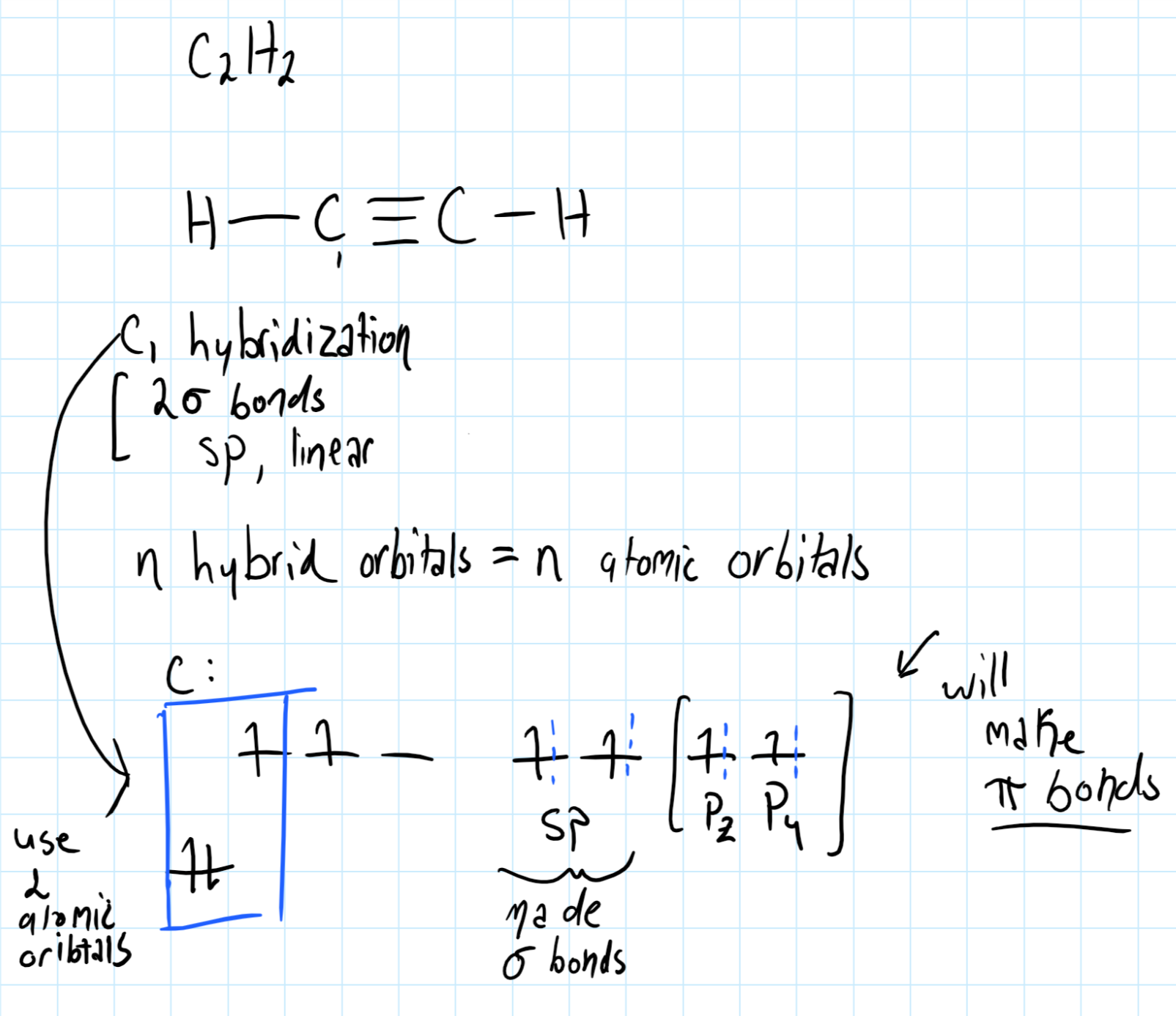
Where the 2s and 2p atomic orbitals in carbon are used to make an
And:
s,p,d,f,g and are used when writing valence electron configuration shorthand, consider below:
Notice how the superscripts for the pertaining element add up to the total amount of the electron in the element, or when written with the noble gas core, the sum of the total superscript value will equal the amount of valence electron in the atom.
An energy level diagram of s,p,d orbitals is also written to the right.