How do you name alkenes using systematic names?
1 Answer
You name alkenes as you do alkanes, but with the ending ene and giving the double bond the highest numbering priority.
Explanation:
-
Find the longest continuous chain of carbon atoms that contains both carbons of the double bond.
-
Give the lowest possible number to the
#"C=C"# double bond. -
Add substituents and their positions to the name of the alkene as prefixes.
-
Identify stereoisomers. It is always safest to use the Cahn-Ingold-Prélog E/Z notation.
EXAMPLE
Give a name for
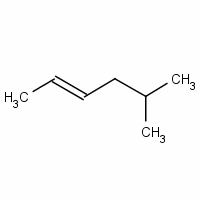
We have a six-carbon chain with a double bond between
The methyl group at
Now we indicate stereochemistry.
The two higher-priority groups are the methyl group on
Since they are on opposite sides of the double bond, the configuration is E.
The full name for the compound is (E)-5-methylhex-2-ene.
(E)-5-methyl-2-hexene is an acceptable name, but the preferred name puts the numbers closest to the groups they locate.

