What is an atom?
4 Answers
Atom is the smallest fundamental unit of matter. All forms of matter are made up of very tiny particle called atoms. Just like human body consists of tiny cells, similarly , atoms make matter around us.
An atom consists of a dense central nucleus surrounded by a cloud of negatively charged electrons.
https://www.khanacademy.org/science/chemistry/introduction-to-the-atom/v/introduction-to-the-atom
An atom is the smallest particle of an element which will retain the properties of that element.
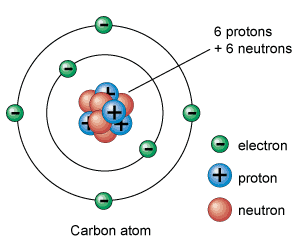
Atoms are made of protons, neutrons and electrons.
The nucleus of an atom is the location where protons and neutrons are found. The electrons are found in a region of space called the electron cloud.
Noel P.
An atom is the smallest particle of an element that has the properties of the element.
An atom consists of a tiny yet relatively massive central nucleus containing at least one proton, and usually one or more neutrons, as well as one or more electrons in the electron cloud surrounding the nucleus. In a neutral atom the number of protons equals the number of electrons.
Neutrons have the greatest mass, and have no charge. Protons have a slightly smaller mass than a neutron, and are positively charged. Electrons have a mass approximately 2000 times less than a proton, but have a negative charge equal in magnitude to the charge of a proton.
It is often loosely described as the smallest building block of matter, and from which elements are composed.
The main sub-atomic particles are the positively charged protons and neutral neutrons which occur in the nucleus of the atom and make up its mass, with the negatively electrons, being about 1600 times lighter than protons and neutrons, circulating the nucleus in different orbits and energy levels and constituting its volume.
The net charge on the atom is usually neutral, but when it is charged, it is quantized and integral multiples of the smallest charge, the charge on 1 electron being
The energy possessed by each sub-atomic particle, which may be considered to have a dual nature, both particle and wave, is also always quantized. (
However, if one studies quantam and particle physics, we see that there are also other smaller sub-atomic particles which can occur, and which may be entangled and which may not always be present in a predictable place and can quantam leap to different spots in their waveform. Examples would be quarks, gluons, muons, photons, leptons, mesons, bosons, etc.




