How can I calculate the bond order of benzene?
1 Answer
Apr 11, 2014
You draw the molecular orbitals. Then you add electrons and count the number of bonding and antibonding electrons.
The bond order of a bond is half the difference between the number of bonding and antibonding electrons.
BO = ½(B – A)
The C-C σ Bonds
Each C-C σ bond is a localized bond. It has 2 bonding electrons and 0 nonbonding electrons.
σ BO = ½(B – A) = ½(2 – 0) = 1
The C-C π Bonds
Benzene has 6 molecular π orbitals.
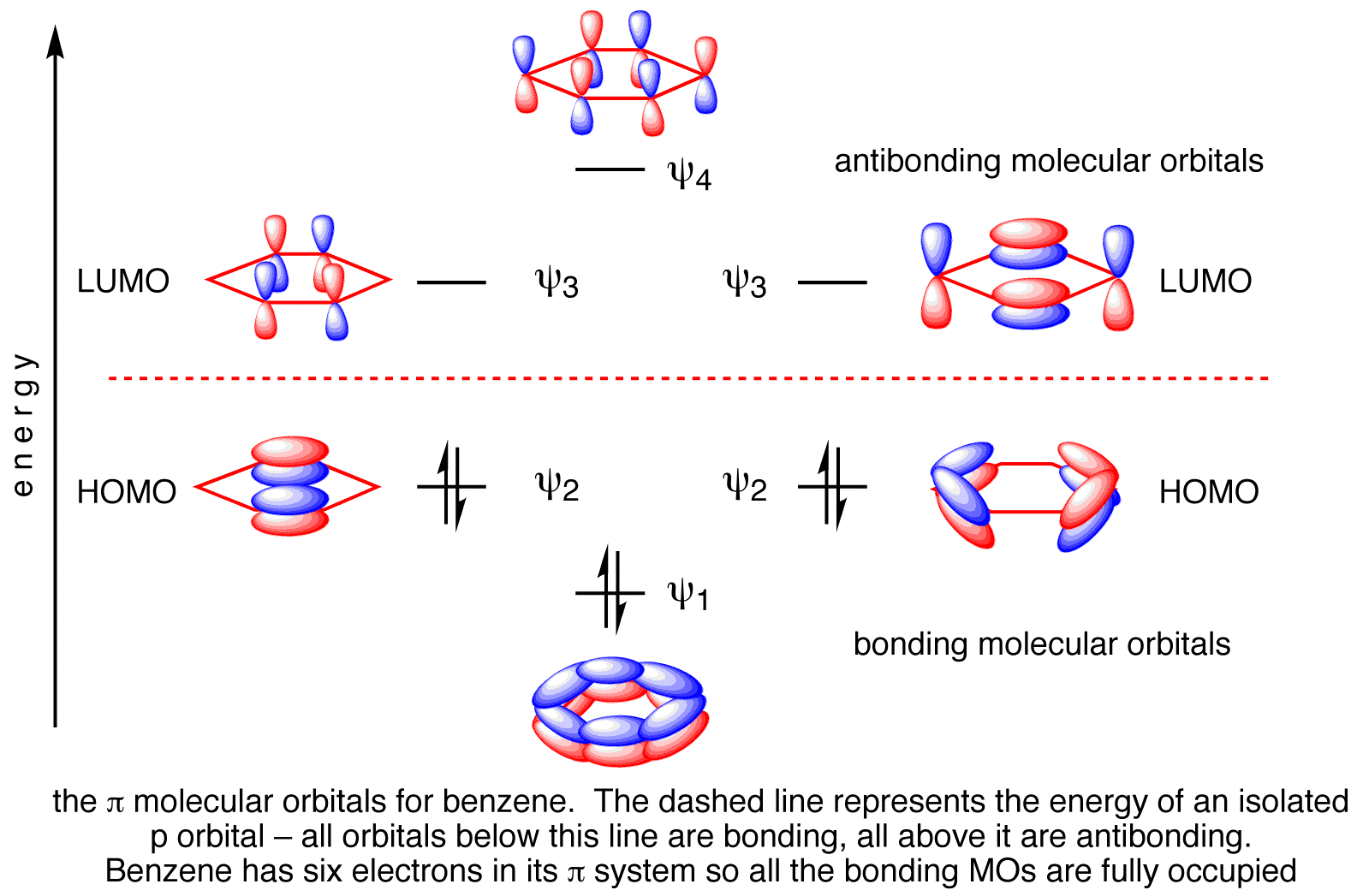 www.chemtube3d.com
www.chemtube3d.com
Of these, three are bonding and three are antibonding. The six π electrons go into the three bonding orbitals.
π BO = ½(B – A) = ½(6 – 0) = 3
This is the π bond order for 6 C-C bonds.
For one C-C π bond, BO = 3/6 = 0.5.
For a single C-C bond in benzene, the total BO = σ + π = 1 + 0.5 = 1.5.

