Why are orbitals described as probability maps?
1 Answer
Because we can't know where the electron actually is, at any time.
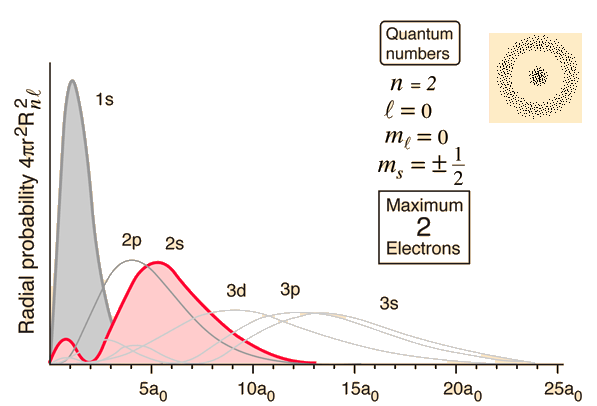
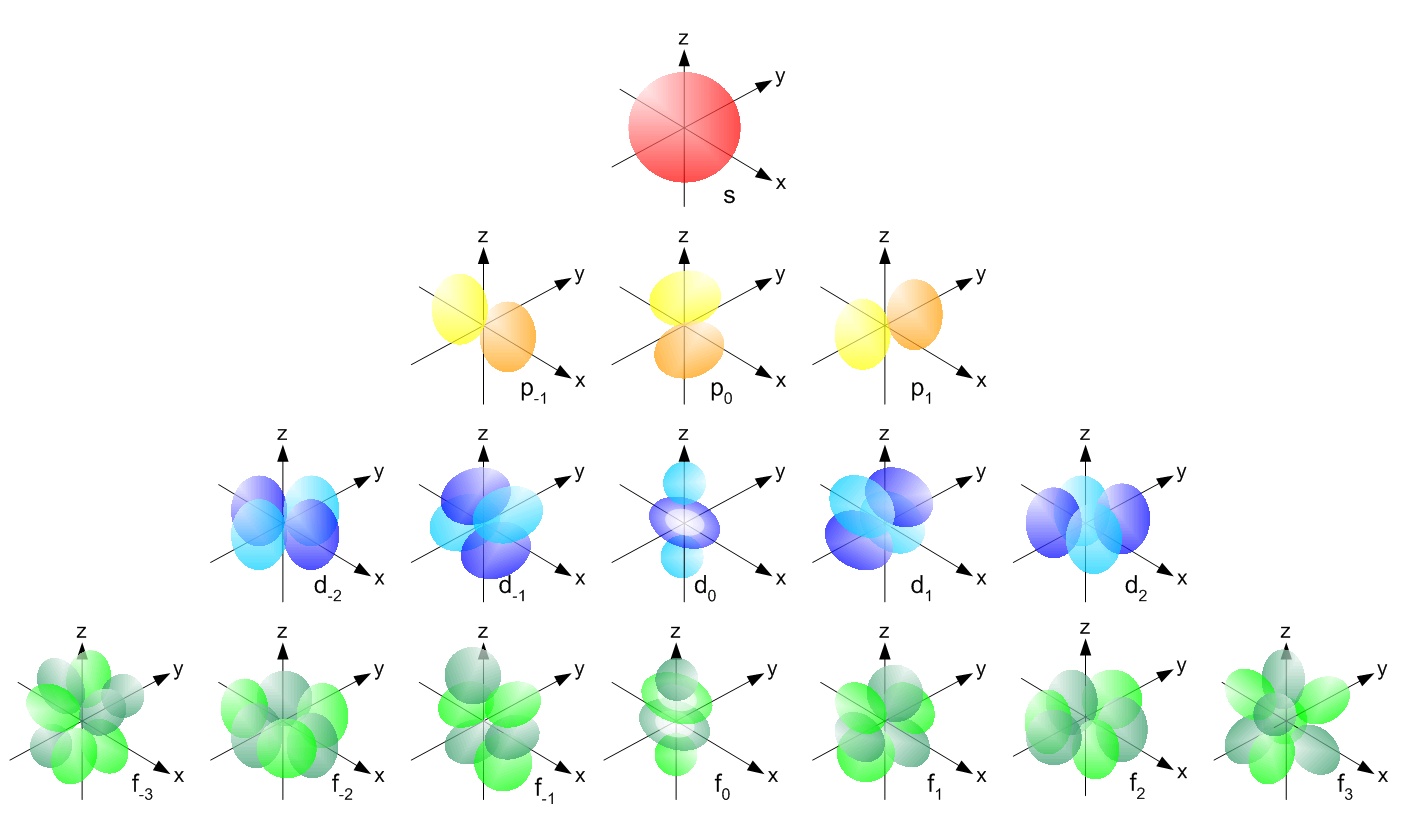
Instead, what we do is calculate the probability of an electron being at each point in the space around the nucleus of an atom. This three-dimensional set of probabilities shows that electrons don't tend to be just anywhere, but are most likely to be found in defined regions of space with particular shapes. We can then choose a level of probability, such as 95%, and draw an edge around the volume where the electron has a probability of 95% or better of being found. These volumes of space are the classic orbital shapes that you will have seen.
Within these spaces, the probabilities are not the same, however, so orbitals are also sometimes displayed as radial distribution functions: graphs plotting probability vs. distance from the nucleus.