How do you solve a gas law stoichiometry problem?
1 Answer
The easiest way is to remember that in order to use stoichiometry, you need to know the moles of the two substances concerned.
Explanation:
We can use the gas laws to help us to determine the effect of temperature, pressure, and volume on the number of moles of a gas.
The central requirement of any stoichiometry problem is to convert moles of
If
If
Here's a flow chart to help you through the process.
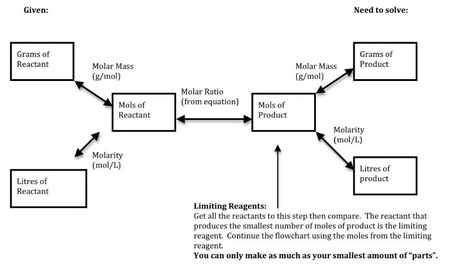
(From homepage.usask.ca)
EXAMPLE 1
What volume of oxygen at STP is produced when 10.0 g of potassium chlorate decomposes to form potassium chloride and oxygen?
Solution
- First, you need the balanced chemical equation for the reaction.
- You then use the molar mass to convert grams of potassium chlorate to moles of potassium chlorate.
- Next, the central part of the problem is to get the molar ratio between potassium chlorate and oxygen. This gives you the moles of oxygen.
- Finally, you use the molar volume to convert moles to litres.
Let’s see how this works.
Step 1. Write the balanced equation.
Step 2. Calculate the moles of
Step 3. Calculate the moles of
The balanced equation tells us that 2 mol
Step 4. Convert moles of
Since 1997, STP has been defined as 0 °C and 100 kPa.
The molar volume of an ideal gas at STP is 22.711 L.
At STP, we use the relation 22.711 L = 1 mol. Therefore
Notice how we always write the conversion factors so that the units cancel to give the desired units for the answer.
If the question asks you to find the volume of gas at some other temperature or pressure, you can use the Ideal Gas Law,
Suppose the question had asked for the volume at 1.05 atm and 25 °C (298 K). You would write
EXAMPLE 2
What mass of potassium chlorate is required to produce 3.00 L of oxygen at STP?
Solution
- First, you need the balanced chemical equation for the reaction.
- Next, you use the molar volume to convert litres to moles.
- The central part of the problem is to get the molar ratio between potassium chlorate and oxygen. This gives you the moles of potassium chlorate.
- You then use the molar mass to convert moles of potassium chlorate to grams of potassium chlorate.
Step 1. The balanced equation is
Step 2. Convert litres at STP to moles.
Step 3. Convert moles of
Step 4. Calculate the moles of
If the question asks you to find the volume of gas at some other temperature or pressure, you can use the Ideal Gas Law,
Suppose the question gave you the volume at 1.05 atm and 25°C (298 K). You would write
Now that you have the moles of
EXAMPLE 3
Ethylene gas burns in air according to the following equation.
If 13.8 L of
Solution
This one requires a little more work, because you have to use the Ideal Gas Law at the beginning and at the end.
- You already have the balanced chemical equation, so your first task is to use the Ideal Gas Law to calculate the moles of
#"C"_2"H"_4# . - The central part of the problem is to get the molar ratio between
#"CO"_2# and#"C"_2"H"_4# . This gives you the moles of#"CO"_2# . - You then use the Ideal Gas Law to convert moles of
#"CO"_2# to litres of#"CO"_2# under the new conditions.
Let’s see how this works. The balanced equation is
Step 1. Calculate the moles of
Step 2. Calculate the moles of
Step 3. Calculate the new volume.
Here's a great video showint the relation between stoichiometry and thr Ideal Gas Law.

