How do intermolecular forces differ from intramolecular forces?
1 Answer
Intramolecular forces are forces that occur within a molecule, while intermolecular forces are forces that occur between molecules.
Let's say, for example, you have a glass of water or
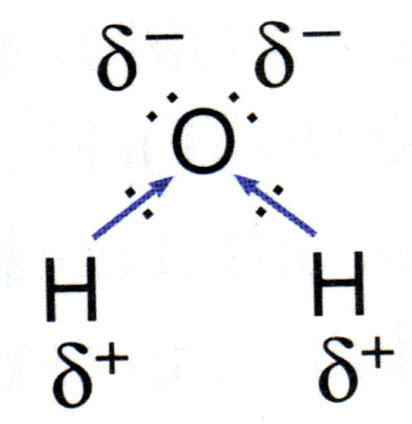
The intermolecular forces are the forces that occur between various water molecules. In the glass of water pictured below, as water is flowing into the glass, the water stays together as a whole. Each water molecule is bound to the ones around it by the intermolecular forces. Intermolecular forces prevent each individual molecule from leaving the other molecules. In water, the intermolecular force of attraction is due to: hydrogen bonding.
There are three main types of intermolecular forces: hydrogen bonding, dipole dipole interactions , and London dispersion forces (previously known as Van der Waals forces).



