What are some common mistakes students make with stoichiometry?
1 Answer
Stoichiometry seems to be a sticking point for many chemistry students.
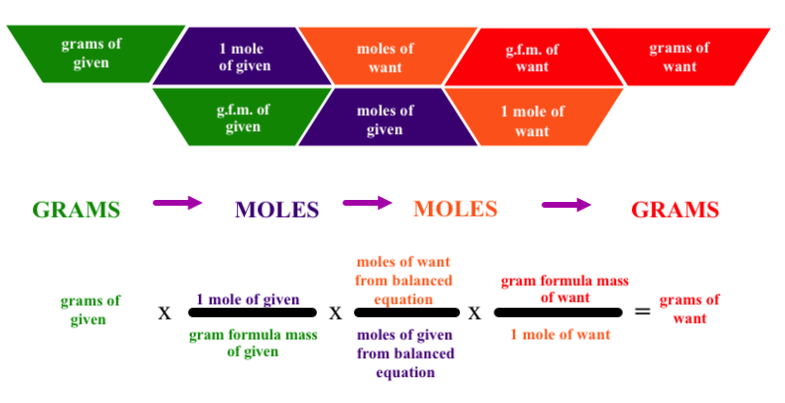
First make sure you have balanced chemical equation with the correct chemical formulas and subscripts in place.
Next identify the knowns and unknowns. Quite often students will not coordinate the correct mass or mole values with the correct products and reactants.
Determine your starting point and ending points to determine the number of conversions that will be necessary.
Next make sure you have calculated your gram formula masses for each of the substances involved in the conversion.
Make sure that you identify the correct coefficients for the mole to mole ratio bridging the known to the unknown.
Be sure to set up your conversion factors so that the units you want to cancel are in the denominator and the units you want to get to are in the numerator.
Make sure you multiply the numerators and divide by the denominators of the conversion factors.
Lastly use significant digits rules to round your answer to the proper value.
 SMARTERTEACHER Computer
SMARTERTEACHER Computer

