How many bonding orbitals in benzene?
1 Answer
Aug 1, 2014
There are 15 bonding orbitals in benzene.
The valence bond (Lewis) structure of benzene is
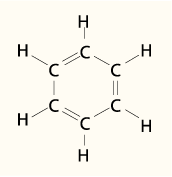
There are 6 C-C σ bonds, 6 C-H σ bonds, and 3 C=C π bonds. This makes 16 bonding orbitals.
According to molecular orbital theory, the σ molecular orbitals form from the three sp² orbitals on each carbon atom and the 1s orbitals on each hydrogen atom.
These 24 atomic orbitals mix to form 24 molecular σ orbitals. Of these, 12 are bonding σ orbitals and 12 are antibonding σ* orbitals.
The 6 atomic p orbitals on the carbon atoms mix to form 6 molecular π orbitals.
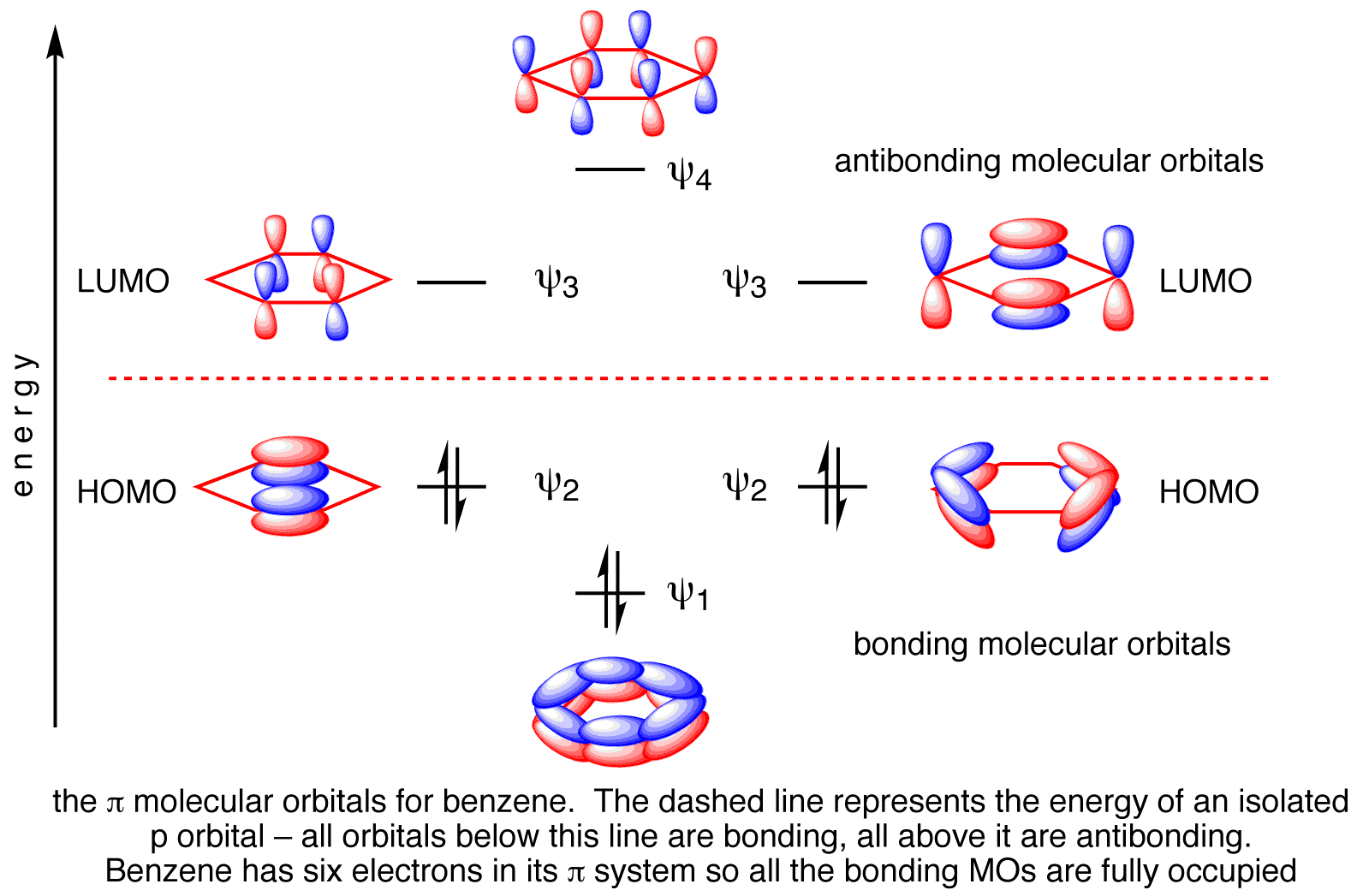
There are 3 bonding π orbitals and 3 antibonding π* orbitals.
So, 12 bonding σ orbitals plus 3 bonding π orbitals give 15 bonding orbitals in benzene.

