How do Bronsted-Lowry acids and bases form conjugate pairs?
1 Answer
When a Brønsted-Lowry acid donates a proton to something else, the particle that is missing the proton is called the conjugate base. Together they are called a conjugate acid-base pair.
When an acid HA dissolves in water, it reacts reversibly with the water to produce H₃O⁺ ions and A⁻ ions.
HA + H₂O ⇌ H₃O⁺ + A⁻
In the process, the HA has donated a proton (H⁺) to the water, and the water has accepted the proton. The HA is a Brønsted-Lowry acid, and the water is a Brønsted-Lowry base.
In the Brønsted-Lowry theory, a conjugate base is whatever is left over after the proton has left. Thus, HA lost a proton to become A⁻, so A⁻ is the conjugate base of HBr.
A conjugate acid is whatever is formed after a base has accepted the proton. Thus, H₂O gained a proton to form H₃O⁺, so H₃O⁺ is the conjugate acid of H₂O.
Thus, HA and A⁻ are a conjugate acid-base pair, and H₃O⁺ and H₂O are a conjugate acid-base pair. A Brønsted-Lowry acid-base reaction always contains two conjugate acid-base pairs.
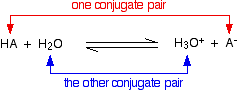 www.chemguide.co.uk
www.chemguide.co.uk

