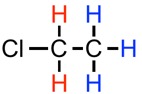
To find the NMR splitting pattern, for a given hydrogen atom, count how many identical hydrogen atoms are adjacent, and then add one to that number. For example, in #CH_2ClCH_3# below, the red hydrogen atoms are adjacent to three identical hydrogen atoms (marked in blue). They will exhibit a quartet (4 peak; 3+1) splitting pattern. For the blue hydrogens, they are adjacent to two identical hydrogen atoms (marked in red), so their splitting pattern will be a triplet.
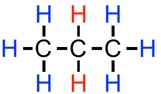
To illustrate this concept with one more example, with propane, #CH_3CH_2CH_3#. Each of the blue hydrogen atoms are still adjacent only to two identical hydrogen atoms, so they will still exhibit a triplet splitting pattern (2+1). This time, however, the red hydrogens are adjacent to six identical hydrogen atoms (with a symmetrical molecule such as propane, all the the blue hydrogen atoms are chemically identical). The resulting splitting pattern will be a seven-peaked septet (6+1).
The physics behind NMR splitting has already been explained in an earlier post.
Sometimes things can get tricky when you have molecules where hydrogen atoms are adjacent to two different sets of identical hydrogen atoms. If you are interested in this more complex situation, please post in the comments.



