What is the dipole moment of nitrogen trichloride?
1 Answer
Nov 8, 2014
The dipole moment of NCl₃ is 0.6 D.
The Lewis structure of NCl₃ is
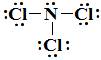
NCl₃ has three lone pairs and one bonding pair. That makes it an AX₃E molecule.
The four electron domains give it a tetrahedral electron geometry. The lone pair makes the molecular shape trigonal pyramidal.
N and Cl have almost exactly the same electronegativities. The electronegativity difference is so small that the N-Cl bonds are nonpolar.

So what is the source of the dipole moment? Answer: the lone pair.
A lone pair will contribute to a dipole moment. Theoretical calculations show the contribution from an sp³ lone pair on nitrogen can be as much as 1.3 D.
So it's reasonable that the dipole moment of NCl₃ is 0.9 D.

