How can I convert L-xylose bond line view to Fischer projection?
1 Answer
Bond-line views give no stereochemical information.
So let's start with the wedge-dash view of L-xylose.
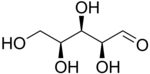
A Fischer projection represents every chiral centre as a cross.
The horizontal line represents bonds extending out of the plane of the paper. The vertical line represents bonds extending behind the plane of the paper.
The Fischer projection of L-xylose is
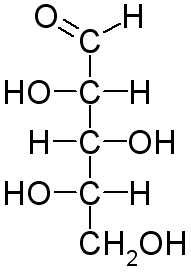
Here's how to convert the wedge-dash structure of L-xylose to its Fischer projection.
Step 1. Arrange the molecule so that the chiral carbons and the longest continuous chain are in a vertical line. C-1 (the aldehyde group) goes at the top.
Step 2. Draw horizontal lines to make crosses at C-2, C-3, and C-4.
Step 3. Put the C-4 OH group on the correct side of the cross.
We must view the molecule from the correct angle.
This is an L-sugar, so the OH must be on the left, and C-4 must be closest to our eye. We must view the molecule from the lower left.
We put the OH on the left arm of the cross. The H atom goes on the right.
Step 4. Put the C-3 OH group on the correct side of the cross.
The OH is on the left, but C-3 is furthest from our eye. We must rotate C-3 to bring it near our eye. The OH then rotates to the right.
We put the OH on the right arm of the cross. The H atom goes on the left.
Step 5. Put the C-2 OH group on the correct side of the cross.
The OH group is on the left, and C-2 is closest to our eye.
We put the OH on the left arm of the cross. The H atom goes on the right.
We now have the Fischer projection of L-xylose.

