What is an antimarkovnikov halogenation?
1 Answer
Jan 1, 2015
An anti-Markovnikov halogenation is the free-radical addition of hydrogen bromide to an alkene.
In the Markovnikov addition of HBr to propene, the H adds to the C atom that already has more H atoms. The product is 2-bromopropane.
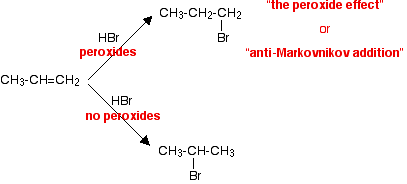
In the presence of peroxides, the H adds to the C atom that has fewer H atoms. This is called anti-Markovnikov addition. The product is 1-bromopropane.

The reason for anti-Markovnikov addition is that it is the Br atom that attacks the alkene. It attacks the C atom with the most H atoms, so the H adds to the C atom with the fewest H atoms.
Here is a video on the anti-Markovnikov addition of HBr to alkenes.

