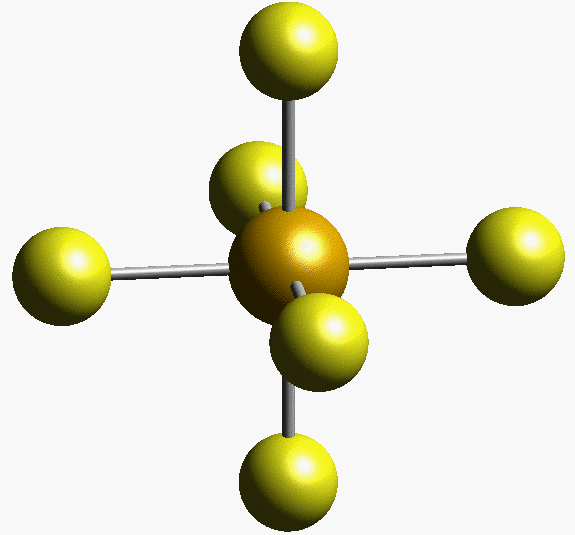
#SI_6#'s molecular geometry is octahedral.
Let's start with its Lewis structure. #S# has 6 valence electrons and each #I# atom has 7 valence electrons, for a total of #6 + 6 * 7 = 48# electrons that need to be accounted for.
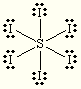
#S# forms six bonds with each #I# atom that account for #6 * 2 = 12# electrons, while the three lone pairs each #I# has make up for the rest - #6 * 6 =36# electrons.
SInce #S# forms bonds with six other atoms and has no lone pairs, its steric number and its coordination numbers are equal to 6. According to VSEPR Theory, the molecule's molecular geometry is octahedral.