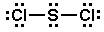#"SCl"_2# has a bent molecular geometry with bond angles of approximately #103^@# and a bond lenght of #"201 pm"#.
Start with the molecule's Lewis structure, which is drawn like this:
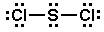
It is important to remember that Lewis structures are not meant to convey geometry, so it would be wrong to assume that the molecule is linear just by looking at this particular Lewis structure.
All 20 valence electrons (6 from #"S"#, and 7 from each #"Cl"# atom) are accounted for by the Lewis structure, so VSEPR Theory can now be used to determine molecular shape.
#"S"#, which is the molecule's central atom, has a steric number equal to 4 and a coordination number equal to 2.
Electron geometry, which is determined by the steric number, will be tetrahedral, while molecular geometry, which is determined by the coordination number, will be bent.
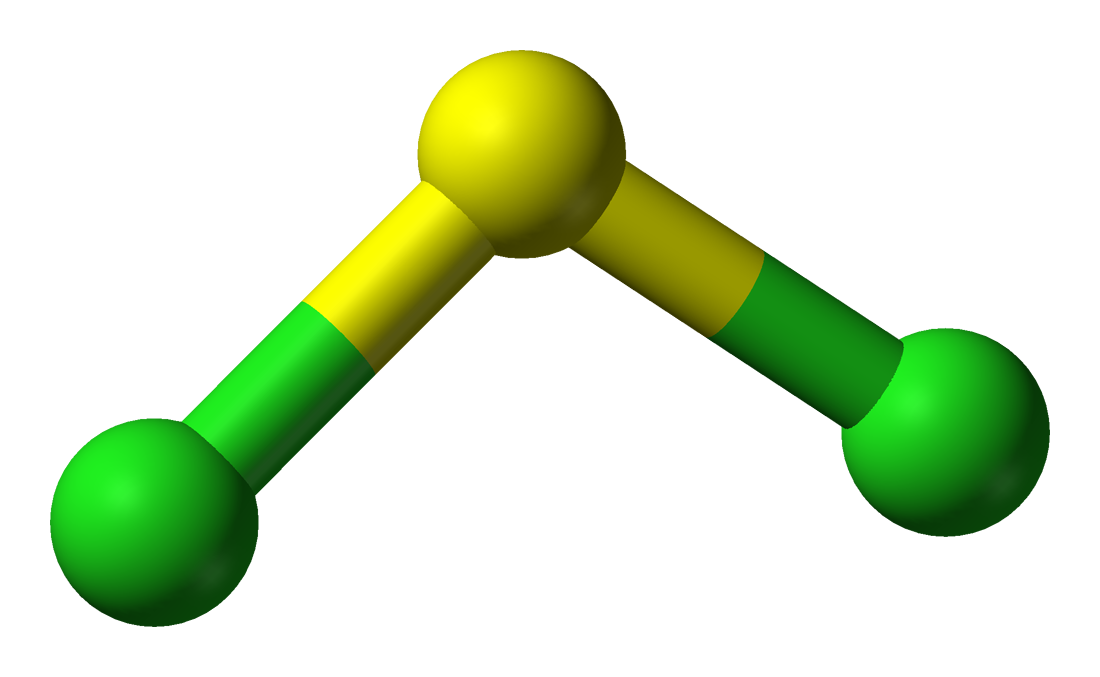
Notice that #"SCl"_2# has a molecualr geometry that is very similar to water's, the only differences being the smaller bond angle (water has a bond angle of #104.45^@#) and the longer bond lenght (water has a bond lenght of #"95.84 pm"#.