Why can't we add a methyl group to to the first carbon atom while making an isomer of butane?
1 Answer
You can add a methyl group to the first carbon atom of a propane parent chain, but that would be equivalent to butane, or normal, unbranched butane.
Here's why that would be so. Below are the two isomers of butane, butane and 2-methylpropane
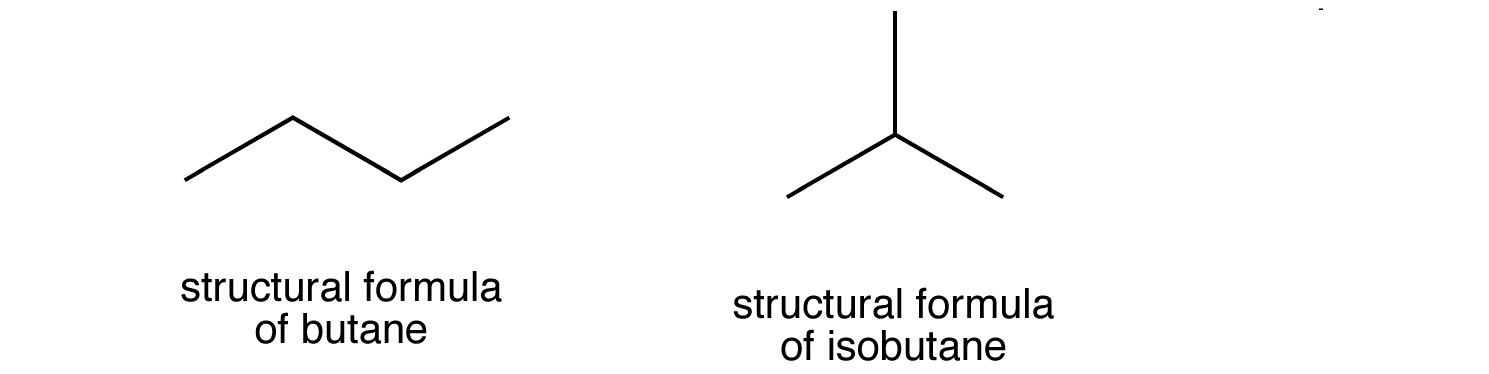
If you start with the bond line notation for propane, or
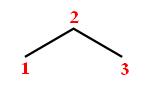
Now, a methyl group is represented as a simple line. If you look closely at propane's structure, you'' notice that placing the methyl group on either carbon 1 or carbon 3 will produce one of these two structures (the methyl group is drawn in blue)
These structures are identical with the first isomer of butane, which looks like this
Therefore, the only way to attach a methyl group to propane in order to make it be a structural isomer of butane is to do it at carbon 2, otherwise you'll end up with unbranched butane again.

