What are the excited states and the hybridization of #"AsO"_4^(3-)# and #"PF"_4^-#?
1 Answer
Mar 2, 2015
They each have an infinite number of excited states. AsO₄³⁻ is sp³ hybridized. PF₄⁻ has sp³d hybridization.
Explanation:
There are three steps to determining the hybridization of an atom:
- Draw the Lewis structure
- Use VSEPR theory to determine its shape.
- Assign the hybridization.
AsO₄³⁻
The Lewis structure is
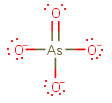
This is an AX₄ system, so it has a tetrahedral geometry.

The steric number (lone pairs + bonds to atoms) is 4, so the hybridization is sp³.
PF₄⁻
The Lewis structure shows a P atom with a lone pair and a bond to each of the F atoms.
This is an AX₄E system, so the ion has a seesaw geometry,

The steric number is 5, so the hybridization is sp³d.

