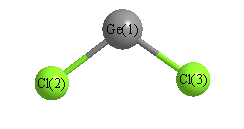How can I predict the bond angles for GeCl2?
1 Answer
You can predict the bond angles of germaniun dichloride,
Start by drawing the compound's Lewis structure. Because it's located in group 14 of the periodic table, germanium has 4 valence electrons.
Each of the two chlorine atoms has 7 valence electrons, which means that the germanium dichloride molecule has a total of 18 valence electrons.
To determine the compound's molecular geometry, you need to calculate germanium's steric number, which represents the electron-dense regions that surround the germanium atom.
In this case,
This corresponds to an