Question #b726c
1 Answer
The formaldehyde molecule has a trigonal planar molecular geometry.
Here's how you'd figure this out. Start with the Lewis structure for formaldehyde,
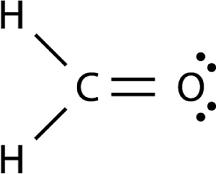
Notice that the central atom, which is the carbon atom, is bonded to three other atoms, two hydrogens and one oxygen, and has no lone pairs present.
This means that its steric number, which represents the number of electron regions that surround an atom, will be equal to 3.
Moreover, the steric number will also give you the orbital hybridization for the central atom. In your case, a steric number equal to 3 will imply the existance of 3 hybrid orbitals, which means that the central atom is
According to VSEPR Theory, an
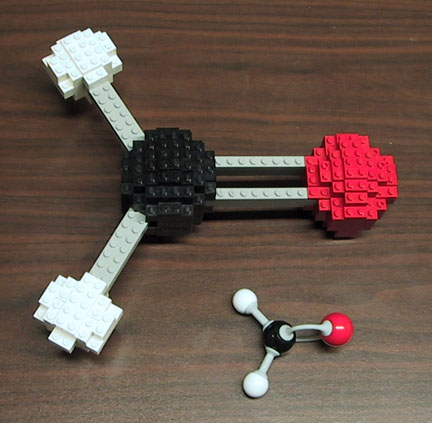
Here's a really cool image of how the formaldehyde molecule looks like