How can I identify dipoles in molecules?
1 Answer
You look for electronegativity differences between the atoms that are bonded to each other.
A bond dipole depends on electronegativity differences (
This causes the electrons in the bond to spend more time around one atom than the other.

For example, in
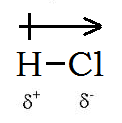
We indicate a bond dipole by an arrow with a "+" at one end and pointing towards the negative end of the bond.
If
EXAMPLE
Identify the bond dipoles on chloromethane,
Solution
The structure of chloromethane is

The


