How do carbohydrates differ from hydrocarbons?
1 Answer
Jun 13, 2015
Carbohydrates contain
Explanation:
The typical general formula for a hydrocarbon is
An example is hexane,
 www.ivyroses.com
www.ivyroses.com
The typical general formula for a carbohydrate is
The name carbohydrate comes from the formula
A typical carbohydrate is glucose,
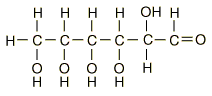 hyperphysics.phy-astr.gsu.edu
hyperphysics.phy-astr.gsu.edu
All the polar
Hydrocarbons are nonpolar and insoluble in water.

