How can I illustrate antimarkovnikov's rule by the addition of hydrogen bromide to propene in the presence of benzoyl peroxide?
1 Answer
You write the mechanism for the reaction.
Explanation:
This is a free radical reaction, so it has initiation, propagation, and termination steps.
Initiation
The chain is initiated by free radicals produced by cleavage of an
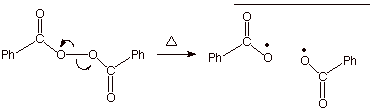 research.cm.utexas.edu
research.cm.utexas.edu
The benzoyl radicals extract a hydrogen atom from an
Propagation
The bromine radical adds to the propene to form the more stable 2° radical.
chemistry2.csudh.edu
The 2° radical reacts with another HBr molecule to produce 1-bromopropane and a bromine radical to continue the process.
chemistry2.csudh.edu
Termination
Eventually two free radicals hit each other and produce a molecule of some sort.
The process stops here because no new free radicals are formed.

