How can I draw two equivalent resonance structures for the formate ion, #HCO_2^-#?
1 Answer
Use a systematic procedure to draw Lewis structures for the ion.
Explanation:
Here are the steps that I follow when drawing a Lewis structure.
1. Decide which is the central atom in the structure. That will normally be the least electronegative atom (C).
2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom:
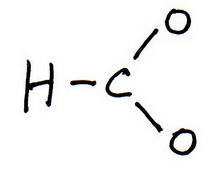
3. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
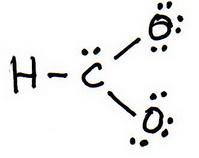
4. Count the valence electrons in your trial structure (20).
5. Now count the valence electrons you actually have available.
The trial structure has two extra electrons.
6. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons.
You have two possible choices for the double bond.
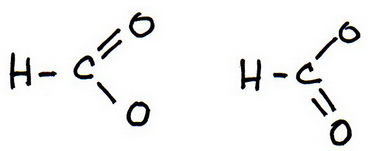
7. As before, add valence electrons to give each atom an octet:
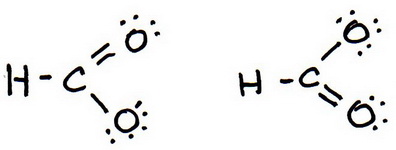
8. Calculate the formal charge on each atom.
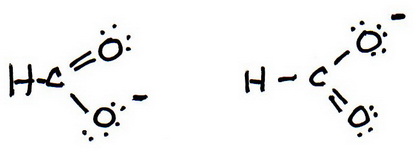
And these are the two resonance structures for the formate ion..

