Question #1ff5c
1 Answer
Methane molecules exhibit only weak London dispersion forces.
Explanation:
Methane molecules are formed when one carbon atoms forms covalent bonds with four hydrogen atoms.
The difference in electronegativity between carbon and hydrogen is small enough that the
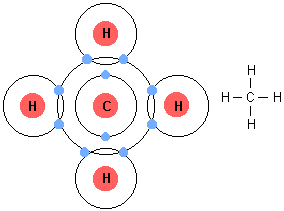
This implies that the bonding electrons are shared almost equally between carbon and hydrogen, no significant separation of charge being formed on the molecule
Even if the
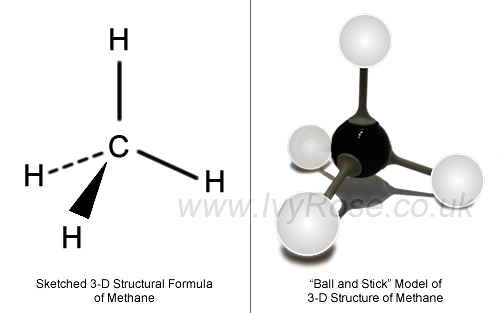
Since the molecule has no permanent dipole moment, the only intermolecular forces it exhibits are weak London dispersion forces, LDF.
London dispersion forces arise when random variations in the electron cloud distribution of one methane molecule polarize the electron cloud of a neighbouring molecule.
Temporary partial charges that appear on one molecule induce similar partial charges in another molecule.
New random partial charges are constantly being formed, the magnitude and orientation varying significantly from molecule to molecule.
Since they depend on random variations in the electron cloud distribution, these intermolecular forces are very short-lived and, as you would imagine, very weak.

