Does “resonance” refer to the idea that molecules are resonating rapidly between different bonding patterns? Why?
1 Answer
See this page for an overview on resonance structures.
Ultimately the term "resonance structure" just means that we are constructing "stills" for molecular states to have more convenient depictions of the molecule that we can use to describe and better understand certain properties and behaviors, like acidity and the molecule's participation in reaction mechanisms. These resonance structures collectively describe the molecule's electron delocalization behavior.
In reality, a molecule with many good resonance structures tends to be described as having good electron delocalization, meaning the electrons are quite mobile throughout parts of the molecule and are shared to a certain extent throughout those parts of the molecule.
Using your wording, the molecule only has one "bonding pattern", and a resonance structure portrays one convenient depiction of the molecule at a stationary point in time. For example:
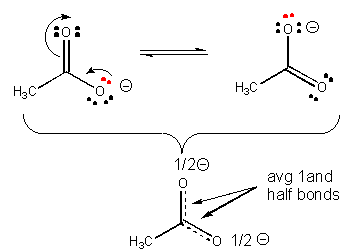
Acetate here has resonance structure depictions for situations when the top or bottom oxygen have the participating electrons close enough to form a

