What are orbital probability patterns?
1 Answer
Once upon a time, you may have imagined that electrons move around in a trace-able way. Really though, we don't know its position if we know its speed and vice versa (Heisenberg Uncertainty Principle), so we only know the probability of finding it at some distance away from an orbital's center.
Another term for "orbital probability pattern" is the orbital's radial density distribution. As an example, the following is the visual radial density distribution of the

...and the following graph describes the probability of an electron being found at a distance
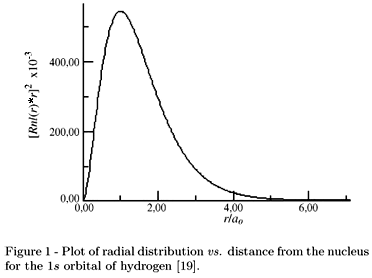
The
(Note that it does not mean more than two electrons are in one orbital, but that an electron shows up however often at however far away from the center of the orbital)

