How are molecular orbitals determined?
1 Answer
It's a bit unclear what you're asking for, but I assume you mean how do we determine which molecular orbitals (MOs) are formed from which atomic orbitals (AOs).
Let's say we looked at methane.
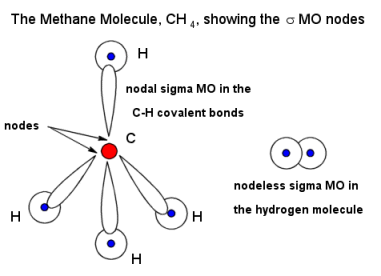
Carbon uses its
The hybridization in carbon can be written out roughly like this:
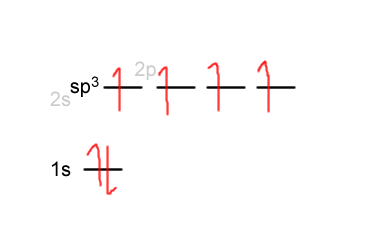
When the orbital overlap occurs, carbon shares its
The overlap between a
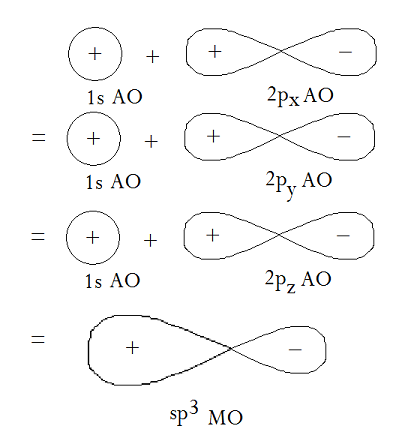
(The carbon is positioned where the
The electron density increases where the
Since a bond must be favorably made to be made often, the resulting MO must support a greater electron density in order to make the bond fairly strong (and therefore stable). Therefore, it is a bonding MO (antibonding MOs are due to opposite-phase overlap, decreasing electron density by creating nodes, and working against bonding, hence "anti").

