Question #e01b7
1 Answer
Explanation:
When you dilute a solution, what you're essentially doing is decreasing the concentration of the solute by increasing the volume of the solution.
A solution's molarity is defined as moles of solute per liters of solution.
#C = n/V#
When you dilute a solution, you keep the number of moles of solute constant. By adding water, which is usually the solvent, you decrease the concentration of the solute by increasing the volume of the solution.
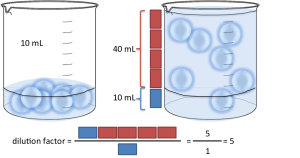
After you perform the dilution, you have the same number of moles of solute in a bigger volume, which is why the molarity decreases.
This is what the formula for dilution calculations means. Let's say that you have a starting solution
#C_1 = n * V_1#
After you dilute this solution, you will get
#C_2 = n * V_2#
Notice that
#underbrace(C_1V_1)_(color(blue)(stackrel("moles of solute")("in the first solution"))) = underbrace(C_2V_2)_(color(red)(stackrel("moles of solute")("in the second solution")))#
As a conclusion, dilution simply means increasing the volume of the solution while keeping the number of moles of solute constant, which results in a smaller concentration.

