A gas occupies 246 mL at 567.3 Torr and 24.0 °C. When the pressure is changed to 771.0 Torr, what temperature is needed to maintain the same volume?
1 Answer
Explanation:
It's always a good idea to start with what you know.
You know that the volume of the container, which is given to you as
This means that only temperature and pressure will change.
When volume and number of moles are kept constant, pressure and temperature have direct relationship - this is known as Gay Lussac's Law.
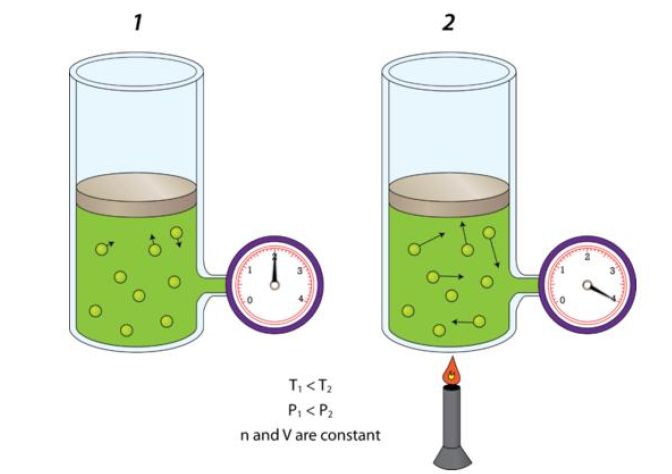
In other words, if pressure increases, temperature increases as well. If pressure decreases, temperature decreases as well.
Mathematically, this is written as
#P_1/T_1 = P_2/T_2" "# , where
In your case, you know that the pressue is increasing from
Plug in your values and solve for
#T_2 = P_2/P_1 * T_1#
#T_2 = (771.0color(red)(cancel(color(black)("torr"))))/(567.3color(red)(cancel(color(black)("torr")))) * 24.0^@"C" = color(green)(32.6^@"C")#
As predict, the temperature must be higher than what it initially was.

