Question #9d55a
1 Answer
Explanation:
You need to write oxygen's electron configuration to help you with that.
Oxygen is lcoated in period 2, group 16 of the periodic table and has an atomic number equal to
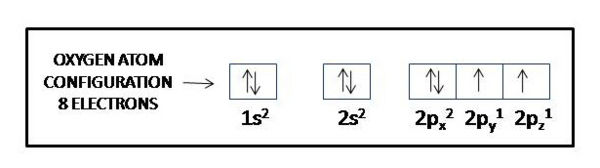
This means that a neutral oxygen atom will have a total of eight electrons surrounding its nucleus. Oxygen's electron configuration will look like this
"O: " 1s^2 color(red)(2)s^2 color(red)(2)p^4O: 1s22s22p4
Valence electrons are located in the outermost energy shell of an atom. In the case of oxygen, its outermost shell corresponds to
You can also write oxygen's electron configuration by using the noble gas shorthand notation, which uses the electron configuration of the noble gas that comes before oxygen in the periodic table.
In this case, helium is that noble gas
"He: " 1s^2He: 1s2
This mean's that oxygen's nmoble gas shorthand will be
"O: " ["He"] 2s^2 2p^4O: [He]2s22p4
Compare this with your set of values
A = 2 ->A=2→ the second energy levelB = 2 ->B=2→ two electrons on the 2s-subshellC = 4 ->C=4→ four electrons on the 2p-subshell
As you can see, oxygen has six electrons in its outermost shell, which is equivalent tosaying that it has six valence electrons.