How many chiral centers does estrogen have?
1 Answer
Contrary to what you might think, estrogen is a type of compound, and sometimes is confused to be a specific compound. There is estradiol, estriol, and estrone, for example. I will take a look at 17
The only chiral centers there can be are those that are
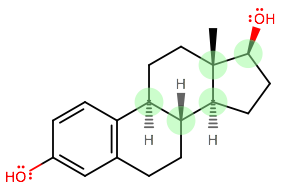
Let's call carbon 1 the bottom left highlighted one, and trace the carbons, ending at the one on the upper right as carbon 5.
CARBON 1:
Substituents = hydride,
Priorities (1, 2, 3, 4):
Stereochemical Label: S
CARBON 2:
Substituents = hydride,
Priorities (1, 2, 3, 4): isopropyl carbon B, isopropyl carbon A,
Stereochemical Label: The reverse of S, which is R (due to the forward hydride, rather than in the rear)
CARBON 3:
Substituents = hydride, tert-butyl carbon,
Priorities (1, 2, 3, 4): tert-butyl carbon, isopropyl carbon,
Stereochemical Label: S
CARBON 4:
Substituents = methyl,
Priorities (1, 2, 3, 4): secondary alcohol, isopropyl carbon,
Stereochemical Label: The reverse of R, which is S (due to the forward methyl, rather than in the rear)
CARBON 5:
Substituents = hydroxide,
Priorities (1, 2, 3, 4): hydroxide, tert-butyl carbon,
Stereochemical Label: S

