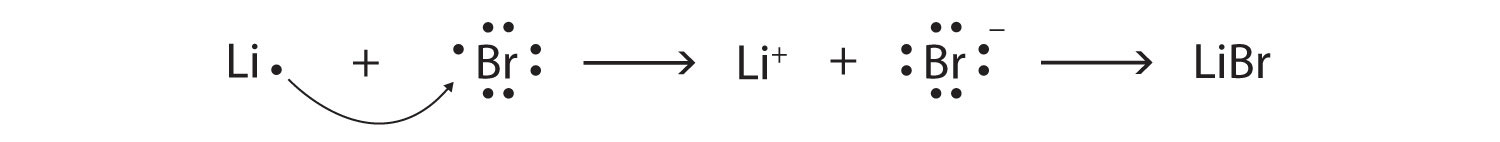How many electrons are there in #Br^-#?
1 Answer
Nov 16, 2015
The
Explanation:
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a
Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons.
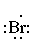
Below is the Lewis dot structure for a
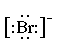
The diagram below shows how a bromine atom gains an electron from the element lithium in order to form the ionic compound LiBr.