What is the unabbreviated electron configuration for argon?
1 Answer
The unabbreviated electron configuration for argon is
Explanation:
The atomic number for argon is 18. The atomic number is the number of protons in the nuclei of the atoms of an element. So argon has 18 positively charged protons, and a neutral argon atom will also have 18 negatively charged electrons.
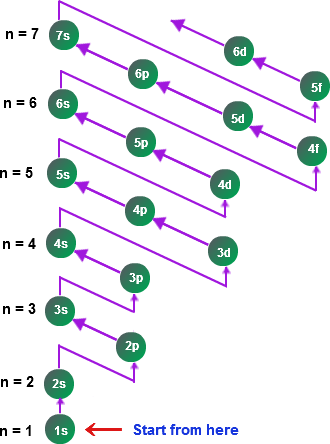
We must follow the Aufbau diagram when filling an atom's orbitals. The Aufbau diagram represents the order of filling of the orbitals in each energy level according to energy, going from lower energy to higher energy.
The unabbreviated electron configuration for argon is
Aufbau Diagram