Question #2b165
1 Answer
Explanation:
Your strategy for this problem will be to use the edge length of the unit cell to determine the unit cell's volume, then use tungsten's relative atomic mass to find the mass of a single tungsten atom.
Once you know that mass of a single tungsten atom, use the total number of atoms that can fit into a body-centered cubic lattice to find the mass of the unit cell.
Since density is usually given in grams per cubic centimeters, it will be useful to start by converting the edge length of the unit cell from picometers to centimeters.
#"1 cm" = 10^(-2)"m" = 10^(10)"pm"#
This means that you have
#316.5color(red)(cancel(color(black)("pm"))) * "1 cm"/(10^10color(red)(cancel(color(black)("pm")))) = 3.165 * 10^(-8)"cm"#
As you know, the volume of a cube is given by the formula
#color(blue)(V = l xx l xx l = l^3)" "# , where
In your case, the volume of the unit cell will be
#V = (3.165 * 10^(-8))^3 "cm"^3 = 3.170 * 10^(-23)"cm"^3#
Now, the unified atomic mass unit,
#"1 u" = 1.660539 * 10^(-27)"kg"#
This means that the mass of a single tungsten atom, expressed in grams, will be equal to
#183.8color(red)(cancel(color(black)("u"))) * (1.660539 * 10^(-27)color(red)(cancel(color(black)("kg"))))/(1color(red)(cancel(color(black)("u")))) * (10^3"g")/(1color(red)(cancel(color(black)("kg")))) = 3.052 * 10^(-22)"g"#
Now, a body-centered cubic lattice is characterized by a unit cell that contains a total of
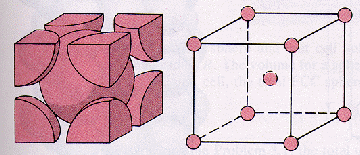
Each corner lattice point contains
#"no. of atoms" = overbrace(1/8 xx 8)^(color(blue)("8 corners")) + 1 = "2 atoms"#
This means that the total mass of a tungsten unit cell will be
#2color(red)(cancel(color(black)("atoms W"))) * (3.052 * 10^(-22)"g")/(1color(red)(cancel(color(black)("atom W")))) = 6.014 * 10^(-22)"g"#
Finally, density is defined as mass per unit of volume. In tungsten's case, the mass of the unit cell and its volume are characteristic of a density of
#color(blue)(rho = m/V)#
#rho = (6.014 * 10^(-22)"g")/(3.170 * 10^(-23)"cm"^3) = color(green)("19.25 g/cm"^3)#
The listed value for tungsten's density is

