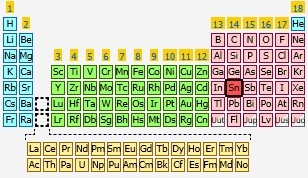
What element has the electron configuration #[Kr]4d^(10)5s^(2)5p^2#?
1 Answer
Tin.
Explanation:
Your goal here is to identify in which block of the periodic table this element will reside.
As you know, the periodic table is divided into blocks, which are sets of neighboring groups.
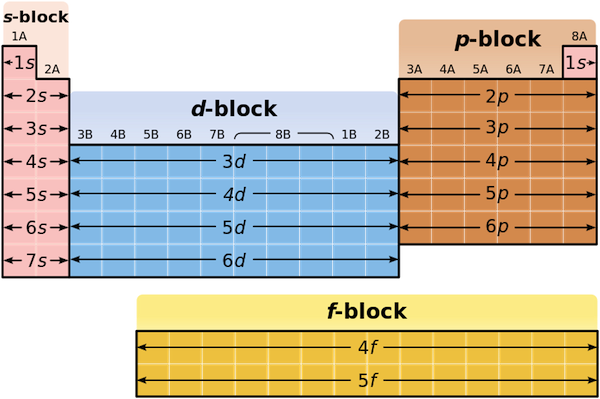
For any chemical element with the exception of a transition metal, its periodic table block will correspond to the orbital type in which its highest-energy electrons reside.
So, you know that you're looking for an element, let's say
#"X: " ["Kr"] 4d^10 color(red)(5)s^2 color(red)(5)p^2#
As you know, an element's noble gas shorthand notation uses the electron configuration of the noble gas that is located immediately before said element in the periodic table.
In this case, you're looking for an element located after krypton,
This is confirmed by the fact that the highest-energy electrons are located on the fifth energy level,
More specifically, this element will have its highest-energy electrons located in a 5p-orbital. Notice that the given electron configuration shows that this element has
This tells you that you're looking for an element located on the fifth row of the p-block, two groups in, since every group corresponds to one electron.
A quick look in the periodic table will reveal that element