In the molecule H_2S, four electron groups around the sulfur atom are arranged in a tetrahedral geometry, but the shape of the molecule is called "bent." Why does the shape have a different name from the name of the electron group geometry?
1 Answer
Here's why that is the case.
Explanation:
You can think about molecular geometry and electron-pair geometry as being two sides of the same coin.
The difference between them is that electron-pair geometry deals with the arrangement of the regions of electron density that surround an atom, and molecular geometry only deals with the arrangement of the atoms that make up a molecule.
As you know, a region of electron density can be
- a single, double, or triple bond
-> all three count as a single region of electron density- a lone pair of electrons**
When determining electron-pair geometry, you count all the regions of electron density that surround a central atom. However, when determining molecular geometry, you only count bonds to other atoms!
In your example, hydrogen sulfide,
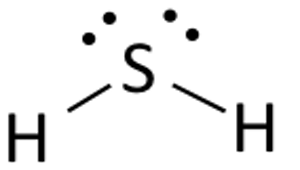
So, how many regions of electron density do you get for the central sulfur atom? This will give you the central atom's steric number.
Well, it is surrounded by
- two single bonds to the hydrogen atoms
- two lone pairs of electrons
This means that sulfur has a steric number equal to
How many bonds to other atoms does the central atom have? This will give you the central atom's coordination number.
Well, since it's bonded to two hydrogen atoms, you can say that its coordination number will be equal to
According to VSEPR Theory, the molecular geometry of a molecule for which the central atom is surrounded by four regions of electron density and is bonded to two other atoms is bent.
Here's an example of how this would look for water,
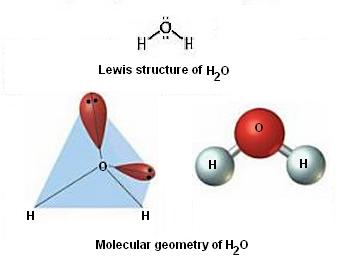
So, to sum this up, electron-pair geometry will sometimes be different than molecular geometry because the former accounts for all regions of electron density that surround the central atom, while the latter only accounts for bonds to other atoms.

