What is an example of a diagnostic test that can be done to distinguish between strong acids and weak acids?
1 Answer
Use a light bulb tester to see if the solution is a good conductor of electricity.
Explanation:
A strong acid reacts almost completely with water to form ions.
In a weak acid, only a small percentage of the molecules are converted to ions.
In an aqueous solution, the ions conduct electricity.
A solution of a strong acid is a good conductor, because it forms many ions in solution.
A solution of a weak acid is a poor conductor of electricity, because it stays mainly as molecules and forms few ions.
We can check the conductivity of the solutions with a conductivity tester.
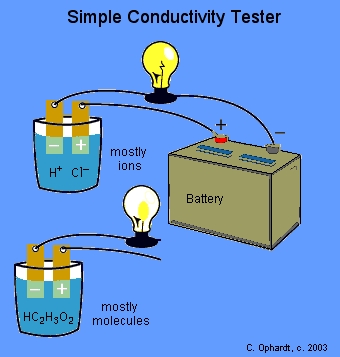
When the circuit is completed by a solution containing a large number of ions, the light bulb glows brightly.
If the solution contains only a few ions, the light bulb glows strongly,
This video shows the conductivity tester in operation.

