Why is density a useful property to know when one wants to identify a substance?
1 Answer
I actually disagree with the question; density is not a particularly good indicator of what a substance is.
CAVEAT 1
For example, quartz and aluminum, at least in this table, have the same density.
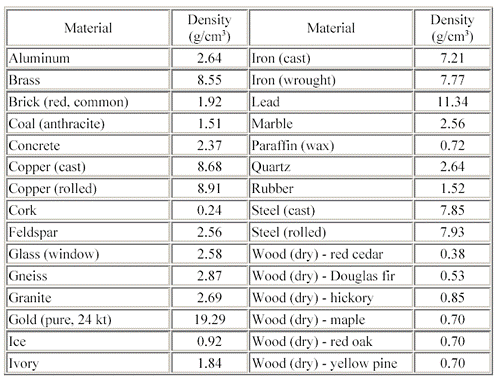
But hardly ever does quartz contain even trace amounts of aluminum. Now, if somehow quartz had the same look and smell as aluminum, I doubt you could tell the difference simply by determining their densities.
You'd need more information, like their behavior in certain reactions for example. Aluminum would react with
CAVEAT 2
You could also say that maybe a metal is an alloy, and you never knew that ahead of time. Perhaps it contains a substantial amount of copper, and maybe you didn't know that. That doesn't tell you what else is in the alloy.
WHOOPS!
So density is really only useful for identification if you already know what the substances are, and you just need quick confirmation for whatever reason.
Maybe someone already knows what the identity of the substance is and is testing you on it, like a professor would for his/her students.
It's a decent indicator, but not great.
WHAT IS DENSITY USEFUL FOR?
1) What it IS useful for, though, is that the density tends to be substantially different for water (
You can depend on density differences to realize that diethyl ether would be the top layer when combining it with water.
That helps during organic separation processes when you want, say, an ionic side product, to drift into the aqueous layer so you can separate it out of the solution.
You still identify which compound is which, because both are colorless liquids, but you don't want to smell organic compounds haphazardly, so the density is nice to know for that reason.
2) Now, if you know density in conjunction with something else, like specific heat capacity, then it becomes pretty useful.
So if you were doing a lab where you had to identify metals that you already knew were pure metals (or you knew which single metal was an alloy), then knowing the density should give you a pretty decent idea, because the professor or teacher probably doesn't want to make it near impossible to figure out.
Then, figuring out the specific heat capacity via heating trials---and knowing the masses, calorimetric heat capacities, and temperature changes---would help you have a better idea of which metal is which when you additionally consider the density. You might do this in a physics lab in the future.

