What is the electron dot structure for PCl3?
1 Answer
Jan 23, 2016
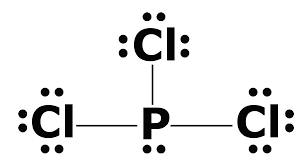
Explanation:
5 steps:
1) Find total number of valence: P = 5 and
2) Always the most electronegative element in the middle: P
3) Use two electron to form a bond
4) Complete the octet on the outside atom
5) If are not able to complete Octets move to the inside to form a double or triple bond
Now I have recreated the image color coding it.
Red is Chlorine with 7 bond so it need 1 electron to complete the Octet. It can do it by forming a bond with Phosphorous.
On the other hand Phosphorous has 5 valence electrons so it use there to bond with the 3 Chlorine atoms. The other 2 electrons on Phosphorous do not participate in the bond making process so they are paired together.

