How many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at a pressure of 12.4 atm and a temperature of 85C?
1 Answer
Explanation:
Your starting point here will be the balanced chemical equation for the reaction between hydrogen gas and oxygen gas, which looks like this
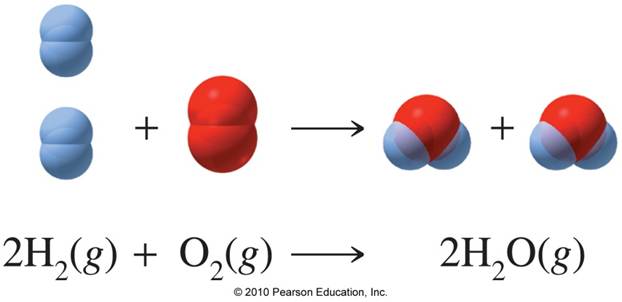
As you can see, you have a
Likewise, you have a
To find the number of moles of oxygen, use its molar mass
#55 color(red)(cancel(color(black)("g"))) * "1 mole O"_2/(32.0 color(red)(cancel(color(black)("g")))) = "1.719 moles O"_2#
You are told that hydrogen is in excess, which means that you can assume that all the moles of oxygen will react.
This means that the reaction will produce
#1.719 color(red)(cancel(color(black)("moles O"_2))) * ("2 moles H"_2"O")/(1 color(red)(cancel(color(black)("mole O"_2)))) = "3.438 moles H"_2"O"#
To determine what volume that many moles of water would occupy at a pressure of
#color(blue)(PV = nRT)#
The value of
Rearrange the above equation to solve for
#PV = nRT implies V = (nRT)/P#
#V = (3.438color(red)(cancel(color(black)("moles"))) * 0.0821(color(red)(cancel(color(black)("atm"))) * "L")/(color(red)(cancel(color(black)("mol"))) * color(red)(cancel(color(black)("K")))) * (273.15 + 85)color(red)(cancel(color(black)("K"))))/(12.4color(red)(cancel(color(black)("atm"))))#
#V = "8.153 L"#
Rounded to two sig figs, the number of sig figs you have for the mass of oxygen, the answer will be
#V = color(green)("8.2 L")#

