Can two #2p# orbitals of an atom hybridize to give two hybridized orbitals?
1 Answer
Feb 8, 2016
No. They are the same in energy, so it would lose the point to hybridize them. They have no necessity to hybridize with other
As a result, if you attempt to hybridize two different
On the other hand, a
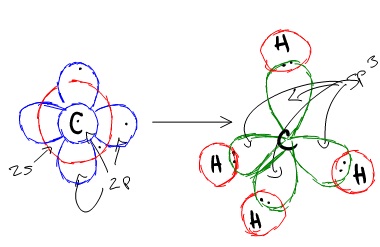
Since methane must make four identical
All four of these

