How to tell if a central element in a molecule needs to form hybridized orbitals?
1 Answer
Simply put, if a central atom has to bond to more than one outer atom, especially if more than one outer atom is different, it has to hybridize.
Diatomic molecules will always point compatible
An easy way to tell when an atom has to hybridize is to count the number of surrounding atoms. I've listed examples below. Essentially:
- Octahedral electron geometry?
#sp^3d^2# - Trigonal bipyramidal electron geometry?
#sp^3d# - Tetrahedral electron geometry?
#sp^3# - Trigonal planar electron geometry?
#sp^2# - Linear polyatomic electron geometry?
#sp#
HYBRIDIZATION IN WATER
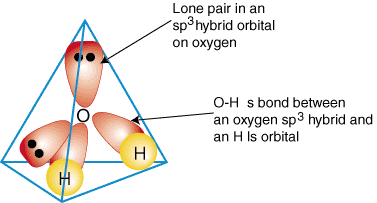
Oxygen in
- It is bonding to more than one hydrogen. That tells you that hybridization can occur.
- It is bonding in a non-horizontal direction to at least one hydrogen. That tells you that hybridization should occur to orient all the orbitals correctly.
- It is bonding identically to each hydrogen. That tells you that hybridization has occurred to make the orbitals compatible.
It is
Oxygen had to hybridize one
HYBRIDIZATION IN BH3
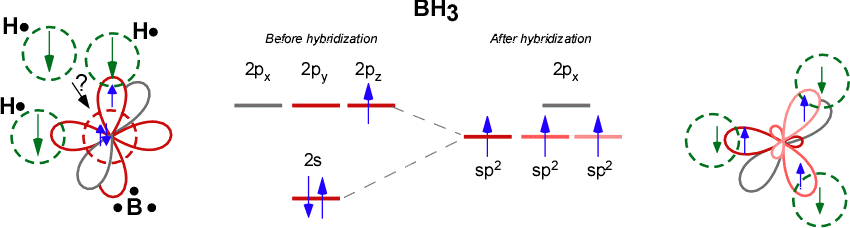
Boron in
- It is bonding to more than one hydrogen. That tells you that hybridization can occur.
- It is bonding in a non-horizontal direction to at least one hydrogen. That tells you that hybridization should occur to orient all the orbitals correctly.
- It is bonding identically to each hydrogen. That tells you that hybridization has occurred to make the orbitals compatible.
It is
Boron had to hybridize one
HYBRIDIZATION IN ACETYLENE
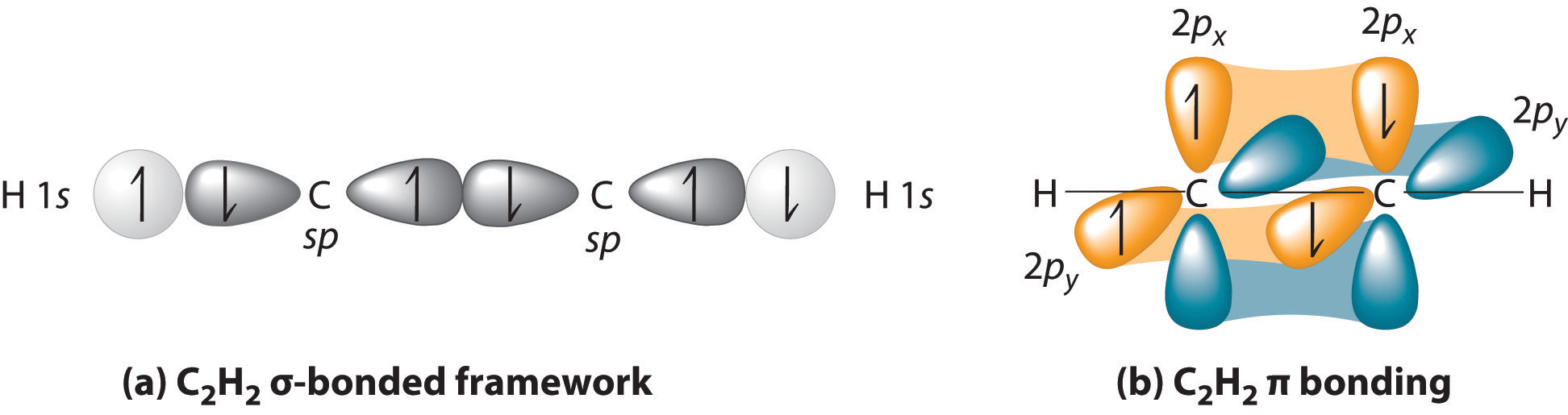
One chosen carbon in
- It is bonding to more than one atom. That tells you that hybridization could occur.
- It is NOT bonding in a non-horizontal direction with any atoms. This doesn't tell you anything about hybridization.
- It is NOT bonding identically to each surrounding atom (the other
#"C"# and a#"H"# ). That tells you that hybridization had to occur to make the orbitals of#"C"# and#"H"# compatible. Naturally,#"C"# is compatible with itself, so hybridization is necessary to bond with BOTH#"C"# and#"H"# .
It is
Carbon had to hybridize one
Separately, the remaining two bonds to be made to the other carbon (one triple bond has one
So, with acetylene, carbon is using two

