Why is the electron configuration of chromium #"[Ar]3d"^5"4s"^1"# and not #"[Ar]3d"^4"4s"^2"#, and why is the #"3d"# sublevel written before the #"4s"# sublevel even though it is higher in energy?
2 Answers
Refer to the explanation.
Explanation:
When writing electron configurations using the Aufbau principle, there are two schools of thought. One is like the electron configuration of chromium in your question, in which the
When written this way, all of the sublevels for
The other school of thought is that the electron configuration of an element should be written so that the sublevels are in order of increasing energy, which will place the
Either way is acceptable, however placing the 3d sublevel before the 4s sublevel is more common. Ask your instructor which method he or she prefers.
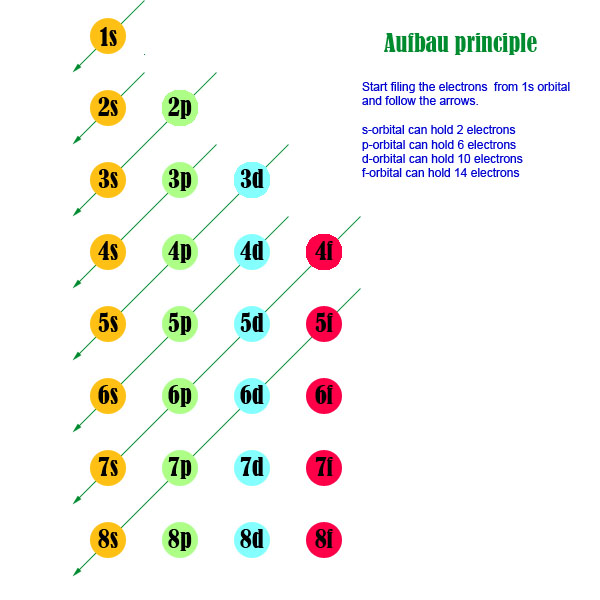
The electron configurations of the elements are determined experimentally. Chromium is one of the approximately 20 transition metal exemptions to the Aufbau sequence of filling orbitals.
Explanation:
The experimentally determined electron configuration of chromium is
It may be that the electron configuration in which a sublevel is completely filled or half-filled is lower in energy and therefore more stable.

