Out of NO+, NO2-, NO3- which has the longest NO bond and which has the shortest NO bond?
1 Answer
The differences here include the number of oxygens attached to nitrogen. Notice the bonding patterns:
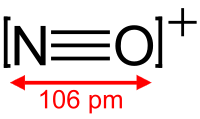
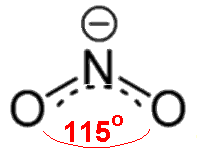
Based on the
Based on the
If you pay attention to the way the oxygens always balance out to give a final charge, notice the following observations:
#"NO"^(+)# , with only one oxygen, has a formal charge of#+1# on oxygen and#0# on nitrogen.#"NO"_2^(-)# , with two oxygens, since there is a#0# formal charge on nitrogen, then the oxygens each share a#-"1/2"# charge (#-1/2xx2 = -1# charge overall).#"NO"_3^(-)# , with three oxygens, since there is a#+1# formal charge on nitrogen, the remaining charge gets distributed onto all three oxygens, balancing out to be#-"2/3"# per oxygen (#-2/3xx3 + 1 = -1# charge overall)
This is interesting, because the oxygens spread out the electron density more and more towards them as the number of oxygens increases.
This spreading out decreases the average electron density within each
When you take the best resonance structure (not the hybrid structure, but the typical resonance structures you draw), you can count one electron per bond to do the following calculations:
- The bond order for
#"NO"^(+)# is#"3 electrons"/"1 bonding group" = 3# . - The bond order for
#"NO"_2^(-)# is#"3 electrons"/"2 bonding groups" = "3/2" = 1.5# . - The bond order for
#"NO"_3^(-)# is#"4 electrons"/"3 bonding groups" = "4/3" ~~ 1.33# .
So, you can see the bond order decreases as the number of oxygens in these
The higher the bond order, the stronger the bond and thus the shorter the bond.
Therefore,

