When is an aqueous solution basic? What pH?
2 Answers
Apr 12, 2016
It is basic at
Explanation:
For any aqueous solution at
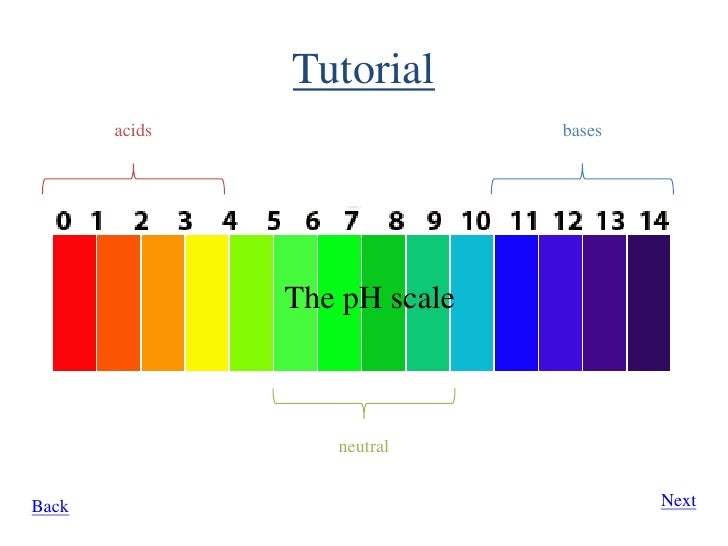
#"pH" = 7 rightarrow "neutral"# #"pH" < 7 rightarrow "acidic"# #"pH" > 7 rightarrow "basic"#
We may note that pH can take any value (even negative values or equal to zero).
P.S. I do not have too much time, but I strongly recommend you to take a look at the definition of pH as the logarithm of
Apr 23, 2016
Basic
Explanation:
The higher the pH number is, the more alkaline (or basic) it gets.
Neutral is 7 so anything higher than 7 is alkaline and anything lower than 7 is acidic.