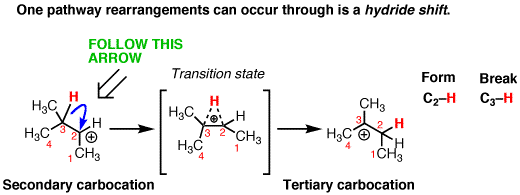
When does a hydride shift occur?
1 Answer
To specify, you meant to ask about a 1,2-hydride shift. Those are taught in organic chemistry, first semester.
When you have a reaction that contains a carbocation intermediate (such as an
CARBOCATION HYPERCONJUGATION
Carbocation stability goes as follows:
#3^@ > 2^@ > 1^@ > "methyl"#
This is due to the
These
The more alkyl groups surrounding the cationic carbon, the more stable it thus becomes, because an additional
1,2-HYDRIDE SHIFTS
Whenever you have a more substituted carbon adjacent to a cationic carbon, a 1,2-hydride shift would shift the positive charge over to a carbon that can better stabilize itself.