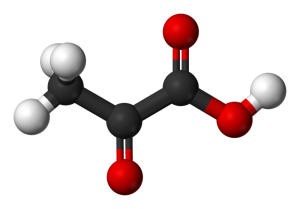
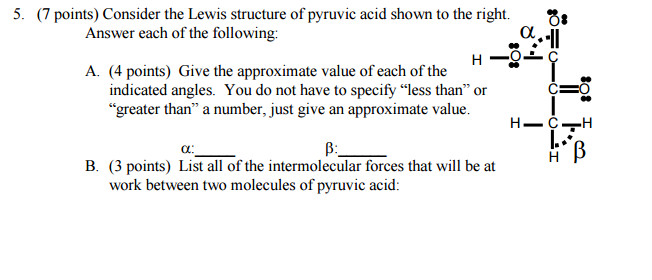
Bond angles and intermolecular forces present?
1 Answer
Here's what I got.
Explanation:
You can give the approximate value of those angles by looking at the hybridization of the two adjacent carbon atoms.
For angle
- a single bond to the oxygen atom that belongs to the hydroxyl group,
#-"OH"# - a double bond to the other oxygen atom
- a single bond to another carbon atom
This means that the carbon atom has a steric number equal to
As a result, this carbon atom will be
For angle
- a single bond to another carbon atom
- three single bonds to hydrogen atoms
This carbon has a steric number equal to
As a result, this carbon atom will be
This can be seen by looking at a molecule of pyruvic acid
Now, a pyruvic acid molecule is polar because of the presence of the carboxyl group and of the carbonyl group,
This means that it exhibits dipole - dipole interactions due to the existence of permanent dipoles.
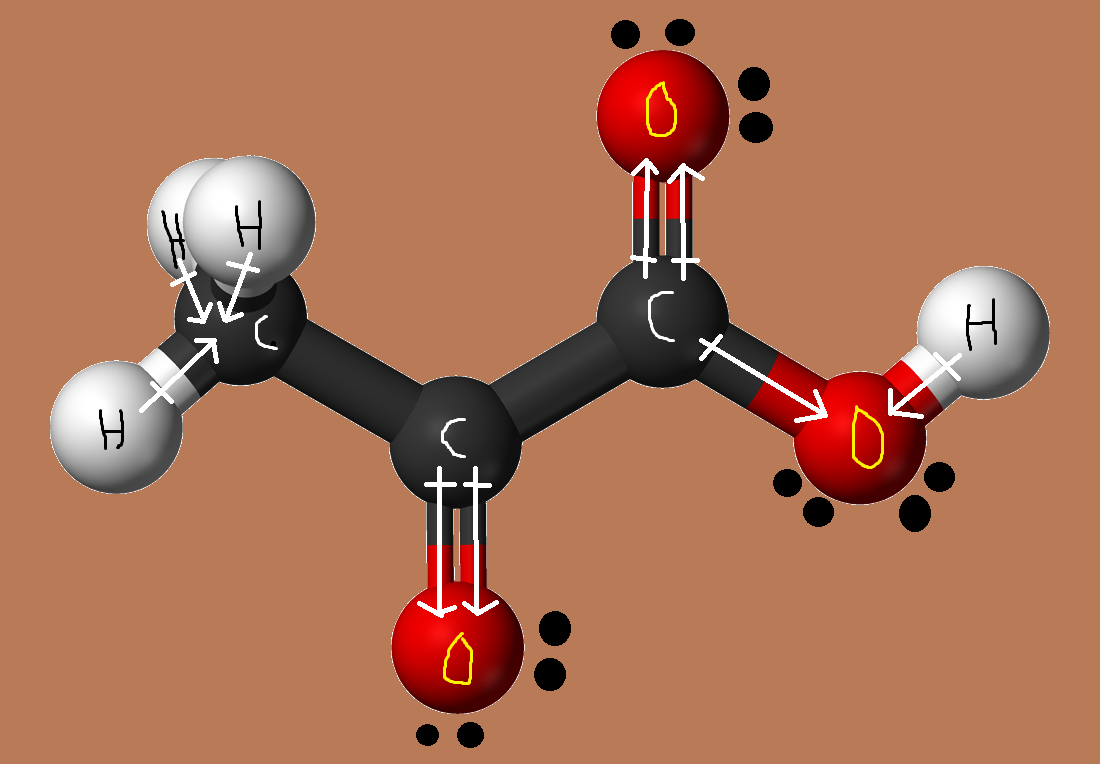
Moreover, because pyruvic acid has a hydrogen atom directly bonded to an oxygen atom in the hydroxyl group, it can act as a hydrogen bond donor.
The molecule can also act as a hydrogen bond acceptor due to the presence of the carbonyl group, which means that pyruvic acid can form hydrogen bonds.
Finally, all molecules, regardless if they are polar or non-polar, exhibit London dispersion forces due to random variations in the distribution of their electron clouds.